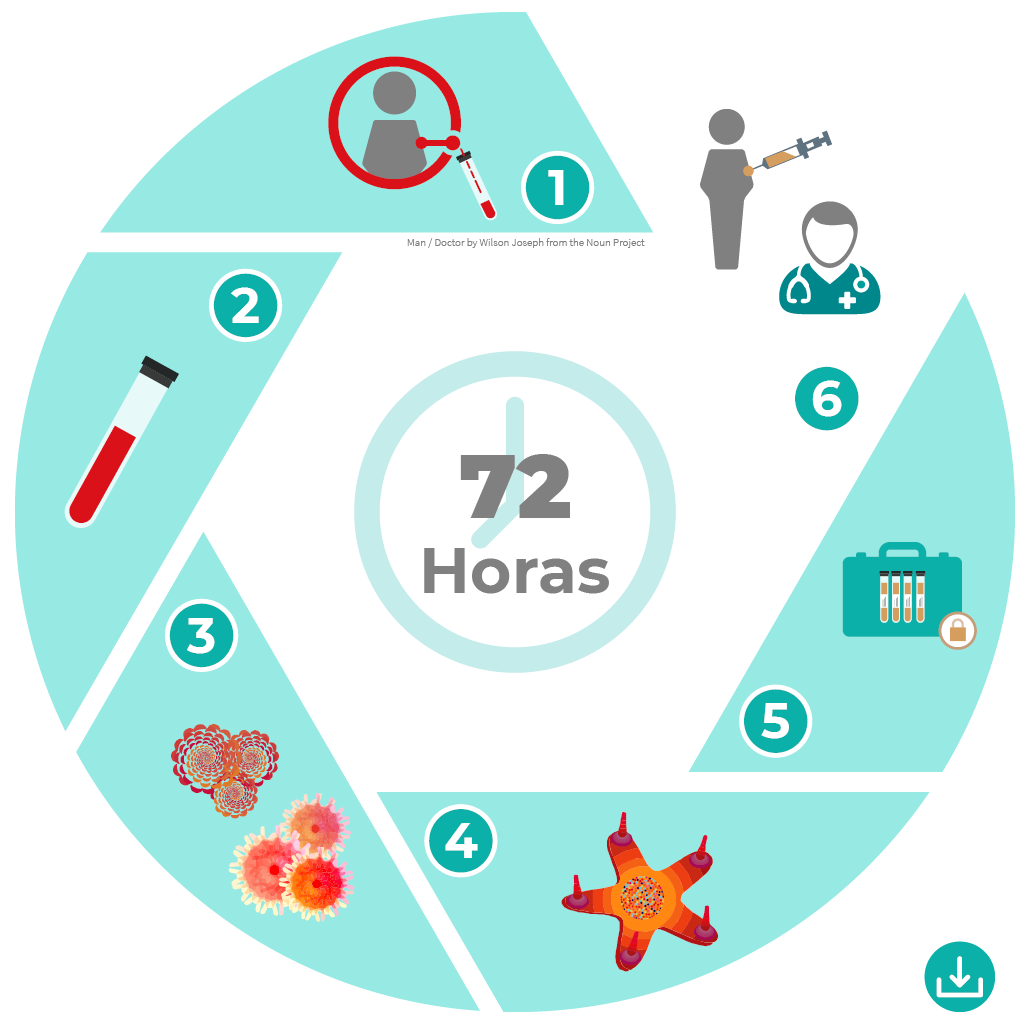
Immunotherapy refers to treatments based on the use of components of the immune system to fight tumours.
TAPCells® therapy was developed by the team led by Dr. Flavio Salazar, at the Anti-Tumor Immunology Laboratory (LIAT) of the Faculty of Medicine of the University of Chile. Oncobiomed has the exclusive license to develop and distribute TAPCells® and is part of several research projects to continue generating technologies for the treatment of cancer, known as cellular immunotherapies..
TAPCells® is based on the stimulation of the patient’s own immune system, so that it reacts against the disease malignant cells.
A scientific and clinical team at Oncobiomed implemented this therapy and made it available for patients, positioning Chile as a regional leader in immunotherapy against cancer.