Treatments
More than 300 patients successfully treated.
More than 20 years of experience in oncology.
Cancer is a disease that is directly related to the immune system, since it is responsible for maintaining the balance between normal cells in the body and eliminating cells that have undergone mutation. However, the appearance of cancer cells alters this balance and occurs when the immune system, for various reasons, is unable to recognise and destroy damaged cells. This causes uncontrolled cell growth, which initially takes the form of a cancerous tumour and can subsequently spread throughout the body.
With the support of the University of Chile and through a medical, scientific and professional team, Oncobiomed has been able to develop technologies to treat cancer safely and without adverse effects, beyond those inherent to any inoculation. These therapies stimulate the patient’s own immune system to generate an anti-tumour response, i.e., the immune system attacks and destroys cancer cells.
TAPCells® technology has been evaluated in phase I and phase II clinical trials in more than 300 patients, successfully fighting skin (melanoma), prostate, colon and kidney cells cancersamong others. The results of this advanced therapy have been recognised globally by the scientific community, the biotechnology industry, and the media.
How does it work?
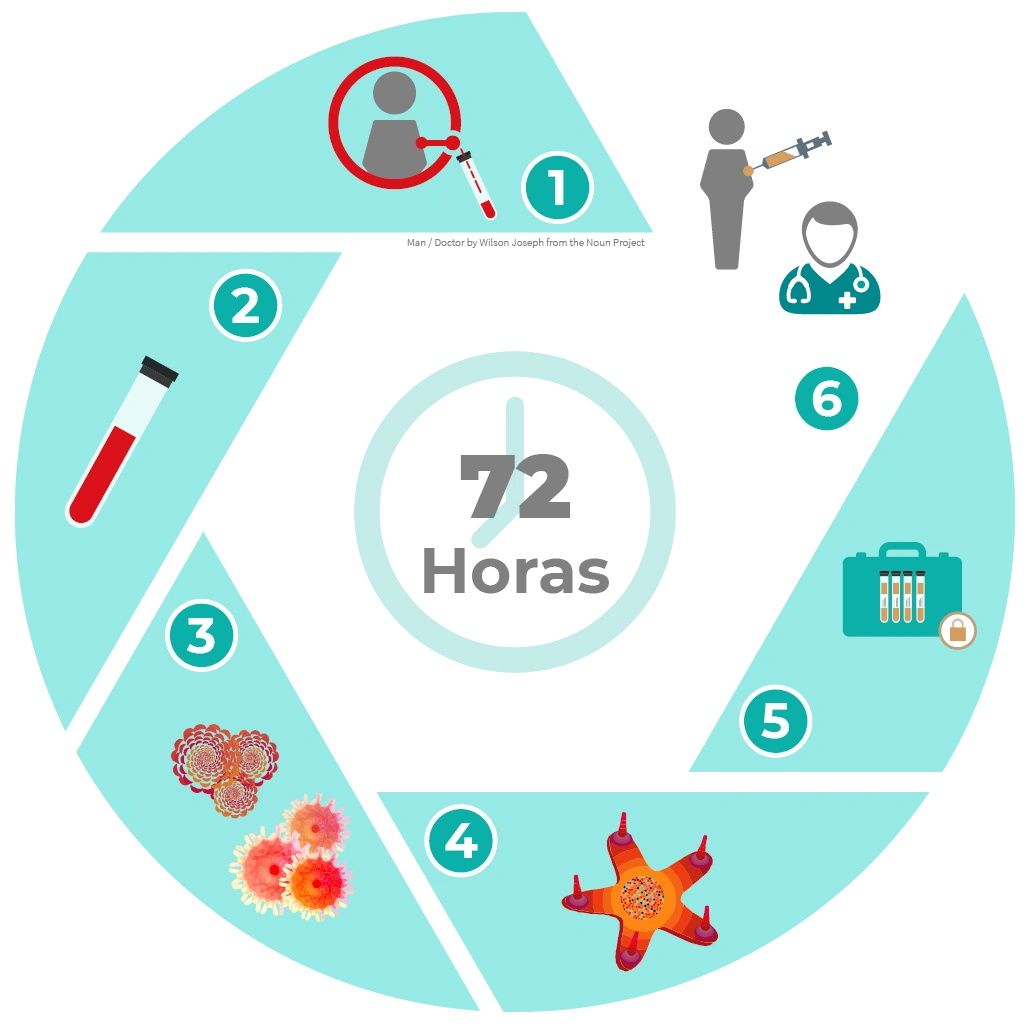
A blood sample is taken from the patient through a process named leukoferesis, from which a monocyte-enriched cell concentrate is obtained.
Monocytes are cultured for 72 hours with cytokines and a specific tumour cell extract for each cancer, enabling to direct the immune response against a given tumour.
TAPCells® doses are stored in the therapeutic kit.
The treating physician applies TAPCells® cell immunotherapy to the patient subcutaneously being integrated to the bloodstream to activate t-lymphocytes, thereby triggering an anti-tumour immune response.
Dr. Salazar is a pioneer in Chile with this technique
Dr. Salazar is a pioneer in Chile with this technique. The vaccine is more than interesting because it gives the immune system the opportunity to recognise the tumour cells based on something that is native to the human body. That is different from what happens with conventional therapies, such as chemotherapy, which in many cases fail to get the immune system working again. If there is a chance of recovery, it is through these treatments.
Juan Carlos Roa
Everything was gone, there were no more tumors
The treatment lasted between March and June 2010. And it worked. First, they told me metastasis had been reduced and then, in September, the scanner showed there was no detectable metastasis. Everything had disappeared, there were no more tumours. I continued with controls every six months until 2014 and since then, I get controls once a year. I’m healthy.
Jaime Lagos
These have been my best years since I was diagnosed
In 2006, I was diagnosed with prostate cancer. After undergoing surgery in the United States and radiation therapy without achieving the expected results, I decided to contact Oncobiomed. Since then, I’ve been able to keep my prostate antigen levels under control safely. These have been my best years since I was diagnosed, as I’ve managed to extend my life without any side effects, unlike other traditional cancer therapies.
José Schiappacasse
I got the vaccine and went on with my normal life
In the midst of the pandemic, at the end of 2020, I was diagnosed with melanoma cancer. After having a tumour removed from my ankle, I contacted Oncobiomed to complement the procedures I was already receiving. The main advantage of this treatment is that there are no side effects and it is a personalised procedure. It is not something they did for the disease in general. They gave me the vaccine and I continued with my normal life, my hair never fell out, nothing.
Constanza Solís
Frequently Asked Questions
1. What is (and what is not) immunotherapy?
It is defined as a treatment based on the administration of immune factors capable of eliciting an immune response, such as vaccines.
As the concept has become common in the clinical market against cancer, it is necessary to distinguish between pseudo-immunotherapies and science-based therapies like TAPCells®, which has carried out clinical studies validated by the Bioethics Committee of the University of Chile; its results have been published in international scientific journals and its application authorised and administered by a medical oncologist according to clinical protocols.
2. ¿Cuál es el respaldo de TAPCells®?
This therapy has been evaluated in a phase I clinical trial and two phase II clinical trials performed in Chile, which results have been published in high impact scientific journals, such as Brit J. Cancer (2013), Cancer Immunology Immunotherapy (2013), Clinical Cancer Research (2011), Journal of Clinical Oncology (2009), Cytokine Growth Factor (2007), Immunobiology (2007), Clinical and Experimental Immunology (2005).
Besides, it has obtained the innovation patent in Chile, Australia, New Zealand, USA, Mexico, Brazil and Israel.
3. What types of cancer are treatable?
TAPCells® technology has been evaluated in Phase I and Phase II clinical trials in more than 300 patients, successfully combating skin (melanoma), prostate, colon, and renal cell cancers, among others.
4. Who applies TAPCells® therapy?
Oncobiomed professionals have technical and scientific competences of the highest level. Patients are evaluated by specialists in oncology who prescribe the treatment if appropriate. A clinical team of specialists in immunology, haematology, oncology and urology evaluate more complex cases.
Vaccines are prepared by biotechnologists and lab technicians who guarantee quality and inoculated by oncology nurses with vast experience in managing oncology patients. A scientific team of the University of Chile monitors technological developments.
5. Since when has this therapy been applied?
Since 2001, over 300 patients have been treated.
6. Are there any side effects?
TAPCells® does not produce side effects, except mild adverse events equivalent to a vaccine inoculation.
7. How important is biosecurity?
TAPCells® production is carried out in accordance with standardised protocols in accredited laboratories for cell manipulation by professionals in the biology field.
8. Is it possible to combine this treatment with others like chemotherapy or radiotherapy?
Current evidence suggests that the combination of different therapeutic strategies produces the best results. Pharmacological inhibitors for tumour growth and antibodies against tumours are now available, besides standard treatments. According to the oncologist’s experience and to each patient’s situation, vaccines can be combined with most of them, not simultaneously, though.
9. What kind of international recognition has this therapy gained?
El reporte internacional de Research and Markets, incluye a TAPCells® entre las inmunoterapias destacadas a nivel global. “Additionally, a wide range of dendritic cell vaccines entered in different phases of clinical pipeline such as Vaccell in Japan, TAP Cells in Chile, MelCancerVac in North America and Rocapuldencel-T in US”.
Roots Analys identifies TAPCells as one of the main anti-tumour technologies based on dendritic cells in the world. ““In fact, two of the three marketed dendritic cell vaccines, PROVENGE® and TAPCells® (Chile), are approved for treatment of prostate cancer”.
10. Is it possible to cure Cancer?
Cancer can be cured by combining different strategies to combat it. For some types of tumors, therapies are available that can eradicate them, while for others, preventive measures have become effective tools for control. Thus, cancer can be cured, but there are no standard treatments with positive results for all patients, due to the individual nature of this disease.
Contact
Contact
Santiago, Chile
+56 9 7753 2325